Lesson: Chemical Bonds and Ions
Chemical Bonds and Ions!


This is an important topic, lesson: chemical bonds and Ions. Bonds and ions are EVERYWHERE on an urban farm. We will need them in BioTechnology, BioEngineering, BioRenewables and BioChemistry. They make everything possible!
Let's start by explaining what each bond does and why. Remember, an ion is NOT a bond, but can lead to bonds. Ions are change agents that put energy into or away from a system.
But First, The Atom!

This is a basic and whimsical diagram of a Helium Atom. There are two protons (+) and two neutons ( ) and two electrons (-). Here the subatomic components are Proton, Pooh, Neutron Eeyore, and Electron, Tigger.
Not all atoms have neutrons. But when they do, they have usually a matching set! All atoms do have protons and neutrons though!
Covalent Bonds
All bonds come down to the electron. The electron zips around the outside orbital of the atom. It isn't always a circle like this, but it is always there.
In a covalent bond, the electron is shared between two or more atoms. in the example below, we have a molecule of water. H2O is the chemical name and it means two hydrogen atoms and one big old Oxygen atom that are sharing Poohs and Tiggers!

I decided to bring all the friends into this diagram. Even though Pooh and Eeyore both live in the nucleus of the atom, they still impact the bonding. That's because atoms are magnetic + and -.
The nice feature of covalent bonds is that it is a casual bond that is easily broken. Like water (H2O) can easily be turned into steam, water or ice. It can be made by igniting Hydrogen in the presence of air, or split using electricity.
Ionic Bonds
Unlike a covalent bond, Ionic Bonds are much stronger. That's because they are LOCKED in an atomic embrace. Think of salt or Lithium Oxide.
We can dissolve salt in water, but it STAYS as salt! That ionic bond is really hard to break apart. That's why I used Rabbit here; He holds onto things with both arms!

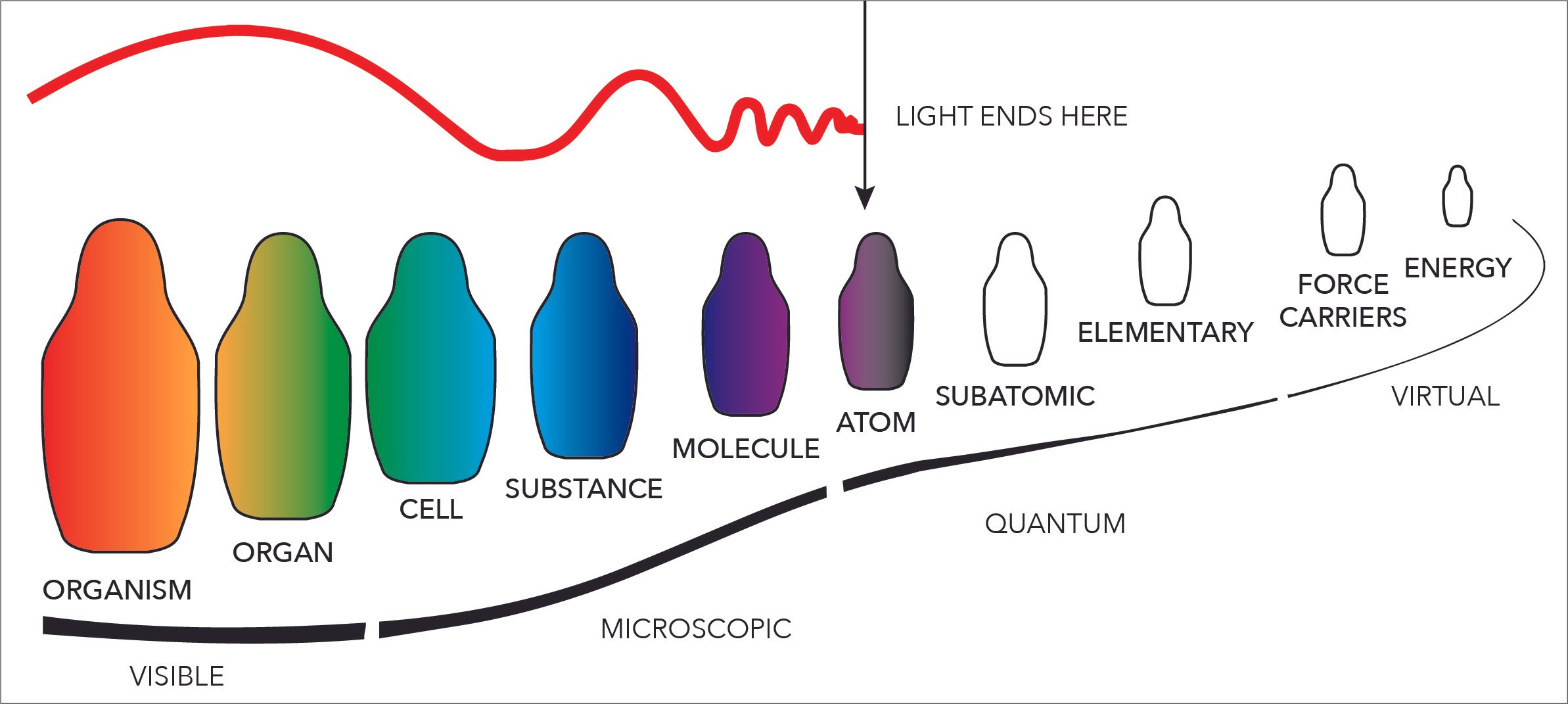
Ionic bonds make more complex molecules and substances possible. Remember what the levels of reality are again. We are FIRMLY in that Quantum and Virtual realm here.
So what is an ion?
An ion is an atom that has an extra or very energetic electron on a level of the electron field that is looking for a magnetic partner. Putting it simply, ions are Tiggers looking for triggers - they want to break things apart or cause reactions to happen. Sometimes they just transfer energy, but something is always happening with an ion around.
In a battery, it's the ions in the Lithium or Zinc Oxide cathodes that push electrons out into the current. The ions are just looking to do something so they aren't so energized anymore. We could not exist if we did not have ions in our body making billions of reactions happen every day.

Next we will talk about the entire FAMILY of atoms in the periodic table of elements.


